The following is a transcript of the presentation video, edited for clarity.
A concept I usually refer to at this point is the idea of the Anna Karenina principle. To paraphrase, the idea is that all happy grants look the same, but there are many, many ways for grants to go awry.
There really is kind of a formula, so I’m going to try to zero you in on that formula.
 You should start to wrap your mind around the application itself. What is the product that you’re putting in? This is where, finally, you get to have your intellectual and creative input into these few pages.
You should start to wrap your mind around the application itself. What is the product that you’re putting in? This is where, finally, you get to have your intellectual and creative input into these few pages.
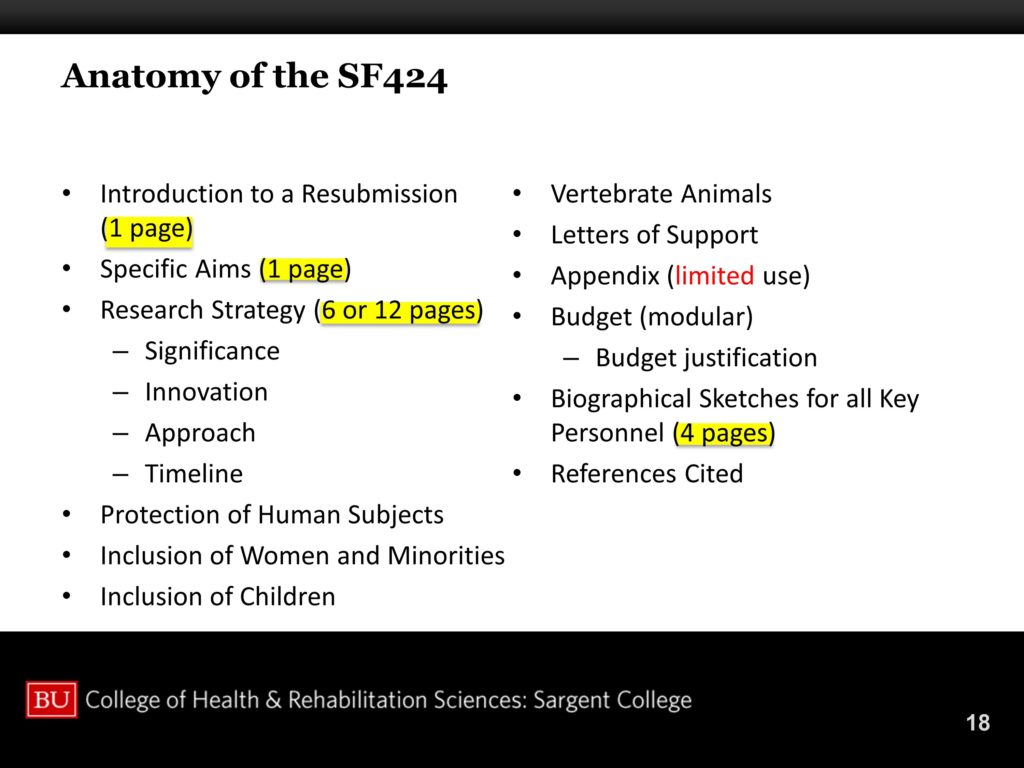
Introduction to a Resubmission
If you’re doing a resubmission, which you usually will. Usually you submit, then usually you resubmit. That’s the course for most applications. Most applications don’t get paid on their first time in. So you get one page to reply directly to the reviewers. And you almost always start that with, “We want to thank the esteemed reviewers for the well-thought comments.” And you make a very gracious, well thought out, non-combative response. Right? It’s not the time to get into a pissing match, because you will lose. They are there, and you’re not.
We already talked a lot about specific aims.
Protection of Human Subjects and Inclusions
You have to make sure you’ve covered protection of human subjects. You can ruin a grant with inadequate attention to human subjects. This is different from your IRB. This is recognizing the four points—and I’m not going to go through them—but there are four points in protections of human subjects: risks, benefits, protections. You have to make sure all of those are covered. It’s not your IRB. IRB approval will be guaranteed, but that’s a local issue. We don’t care about your consent forms, we don’t care about any of those. Those are guaranteed by your local IRB. But we do care that all these protections are in place.
Inclusions absolutely have to be there. You should not save that for the last minute. All these numbers have to add up. If you have all these different racial and ethnic minorities included, they have to add up at the bottom. Like these complicated charts you have to figure out. Those are congressionally mandated, so get someone who’s done that before—don’t try to figure it out by yourself. But it’s required.
You have to include children, which for purposes of NIH children are under the age of 21, not 18. You have to explain why you’re not including children. If you’re including some children— like including 18 to 21 is very common to include those children because you’re running your undergrads—then you’re including children, but you still have to talk about why you’re not including children below 18. It’s fine, you just have to cover that base.
It’s also parallel with vertebrate animals. There are various assurances you have to provide in the care and use of vertebrate animals.
Letters of support we talked about a lot.
Appendix and References Cited
Appendix. Really, you can’t do much with the appendix. It’s a vestigial organ from grants gone by. The appendix used to be a place where you could stack in all kinds of stuff. The problem is, when the page limits got cut in half, from 25 to 12 pages, people just started shoving everything that used to be in those other 13 pages into the appendices. That’s not where they belong, they were using it to circumvent the limits. We’d commonly send grants back, unreviewed, for violating the appendix guidelines.
About the only thing you can put in there would be things like, the actual test itself. That would be a non-standard test. If it’s a test that’s commonly available, you just name the test. You don’t have to include a copy of the test. You can, but it’s just irritating. But a test that isn’t commonly available. A manuscript that is published or in press, but not available. There aren’t very many of these.
It’s worth mentioning that you cannot use any links in your application. So another common problem is people will refer to links, the URLs, in their application. It’s very tempting to do that. All the grants are distributed electronically anyway, you might as well put in a hyperlink. But we explicitly warn reviewers not to go to hyperlinks because ultimately they’re all trackable, so it violates the anonymity of the application. It can be used to exceed the page limitations. It puts on an unfair reviewer burden. So, you can’t depend on links, you shouldn’t include them.
That said, it’s really wonderful in your references cited when you can put in the DOI. You put that in and let people click through the references that way, that’s awesome. Those go out to public databases, and those are publicly available references, and you’ve made it much easier for reviewers so they love you for it.
Biographical Sketches
If you haven’t started working on your new biosketch—it’s really PHS398, submitted through SF424—you should do that. That thing takes a long time. If you have your existing biosketch and you haven’t changed it over yet, start working on it now. It’s challenging. But for now, the thing it really forces you to do, I think, is to go through and create your PubMed bibliography.
The thing you have to recognize about the PubMed bibliography is that you really can’t put anything in that that you can’t tie to a grant, a federal grant. The thing that motivates PubMed, that requires PubMed is that federal funds have paid for research, and the idea is that research needs to be made available to taxpayers. That’s PubMed’s intent. So it can be difficult to upload things, especially for new investigators who haven’t had things that were directly funded. But if you were on a training grant, if you were on an R01—you know, if you worked as a research assistant on an R01, you can tie your work to that grant sometimes reasonably. So, it’s not really a problem to stretch a little bit to tie your work to previous federal funding. I’d encourage you to do that.
There are some others, I can’t remember off hand, but there are other electronic databases are that are allowed in the new biosketch. PubMed is the most limited one, but there are others.
Audience Question: Is it valued to cite your own work that is under review? Or is that the same as it just not being published?
That’s a great question. Under review is a tricky category. For early stage investigators, you can do it. For more established investigators, it’s actually forbidden. When you put it in the running text, I think you can get away with it. But you can’t put it in your biosketch, that’s where it gets problematic. It’s good in the aims to say, “recent preliminary experiments in our lab have demonstrated this.” Recognizing that it’s unsubstantiated and un-peer reviewed, you can’t lean on it too hard. But I don’t have a problem with putting it out there. For a K award, for example, that would be great—if you have those preliminary results, you’re going to put those in your research plan anyway.
Also, there’s such a long time between the submission deadline and the review. It happens all the time where you have a manuscript that’s under review and gets accepted between the time you’ve submitted and the time the manuscript is being reviewed. For fellowships, it’s particularly important that you notify your review officer of that. When you go on Commons, one of the people listed on the Commons page is your review officer. And you can shoot them an email. I actually like to get a PDF of the accepted paper, and a letter or email from the editor indicating acceptance. Because people aren’t always honest about this, so it’s nice to have the evidence. So, of course you’re all honest. But you have to recognize it’s a competition, and there are people out there that will be dishonest. So don’t make the grant review officer come back to you and say, “would you please provide documentation that this is, in fact, true.”
Research Strategy

The research strategy, that’s the six to twelve pages. For an R03 you only get six pages, for an R01 you only get twelve pages. Don’t try to cheat with the font. Don’t try to cheat with the margins. Don’t try to crowd it all in there so it’s punishing to read. Make sure you have enough white space so it’s legible and readable and easy on the eyes.
It has to do a lot of different things. I would just refer you to this list as a starting way to think about them. The narrative used to be something that had prescribed parts, introduction, preliminary studies, research plan. That’s not the case anymore. Those six or twelve pages are very free-form. You still want to include those elements of background and research plan and things we talked about yesterday fully fleshed out. But how you do it is kind of up to you. If you want to include preliminary studies as part of the background. If you want to integrate things a little bit more, you can. They tend to follow much more traditional organization, though, where it’s introduction, research plan, and always ending with a timeline.
Do not forget to do this timeline. It’s not mentioned in the application guide, but it’s pretty much a required element. Including, in the timeline, plans for dissemination along the way.
See if there’s anything else I have to add—feasibility I think I mentioned. Oh, synthetic data. A great thing to do: if you have feasibility, but you don’t have enough data to show how you’d treat the data, you can just create a synthetic data set and run the data. You’re telling them it’s synthetic, that you made up this data, and ran it completely through the analysis, and here’s what the results showed. It’s awesome. That is so compelling. You can show a graph or two or three graphs that show alternate outcomes, how they’ll be interpreted, what the statistics were that got you there. The experiment’s all done. All you have to do then is fill it in with real data. That’s very, very compelling. You see grants that do that and it’s like you’ve gone into another world, it’s just so well thought out. And feasibility in that case is not even a question. All you have to know in that case is how long does it take to acquire and process and finesse the data to get it into the form you need.
Preparing for Your First NIH Grant: More Videos in This Series
1. Who Is the Target Audience for Your Grant?
2. How Do I Determine an Appropriate Scope, Size, and Topic for My Research Project?
3. Common Challenges and Problems in Constructing Specific Aims
4. Are You Ready to Write Your First NIH Grant? Really?
5. Demystifying the Logistics of the Grant Application Process
6. Identifying Time and Budgetary Commitments for Your Research Project
7. Anatomy of the SF424: A Formula for NIH Research Grants
8. Common Strengths and Weaknesses in Grant Applications





