The following is a transcript of the presentation video, edited for clarity. Additional presentation slides can be downloaded by clicking the PDF button in the toolbar.

I will talk to you a little bit about our origin and research pathway, and I’m primarily going to focus on our adventures in implementation. I say adventures very strongly because we didn’t know what we were doing when we started scaling up. And I’ll tell you exactly what we did. So full disclosure about everything. And I’ll tell you about what we’re trying to do today and share some perspectives.
That’s my plan of action.
Let me give you a bit of our story.
NIH has told us to improve human health, scientific discoveries must be translated into practical applications. That’s really at the heart of what we’re all trying to do today.
Parkinson disease is a progressive, neurodegenerative disorder with no known cure, which affects nearly 6 million individuals globally. Over 89% of these individuals have a speech or voice disorder. Vocal loudness, hoarse voice, monotone, and imprecise articulation are among the classic features that contribute to lifelong frustration, embarrassment, and social isolation.


Unfortunately, classical medical treatment alone, such as pharmacological treatment or surgical treatment, do not consistently or significantly improve speech in Parkinson disease. In addition, voice and speech disorders in Parkinson’s have been historically unresponsive to speech treatment. This is a really critical point. It’s not that people haven’t attempted to improve speech in these patients, there’s a long literature of efforts. It’s really difficult to make lasting changes in speech production in Parkinson’s.
Back when we began our work, back in 1987, there was no effective voice and speech treatment for Parkinson’s. And as this client summarizes, “If I have no voice, I have no life.”
So this is a big problem, and people have tried to solve it for many, many years. And we tried as well.
The Research Pathway

The origin and research pathway began with Mrs. Lee Silverman. She was a woman with Parkinson disease. And while she was in a wheelchair, had many other medical problems, her family basically said to me, if only we could hear and understand her. That was the origin and the original impetus for what has now gone on to be LSVT. Over 20 years and good funding from federal and foundation sources allowed us to do Phase I and II trials, multiple baseline single-subject designs, small group studies, etc.
A number of Phase III trials, efficacy trials funded by the NIH. And more recently moving into Phase IV and Phase V, embracing technology both for training and delivery of treatment and scaling up access. So, that’s our research pathway.
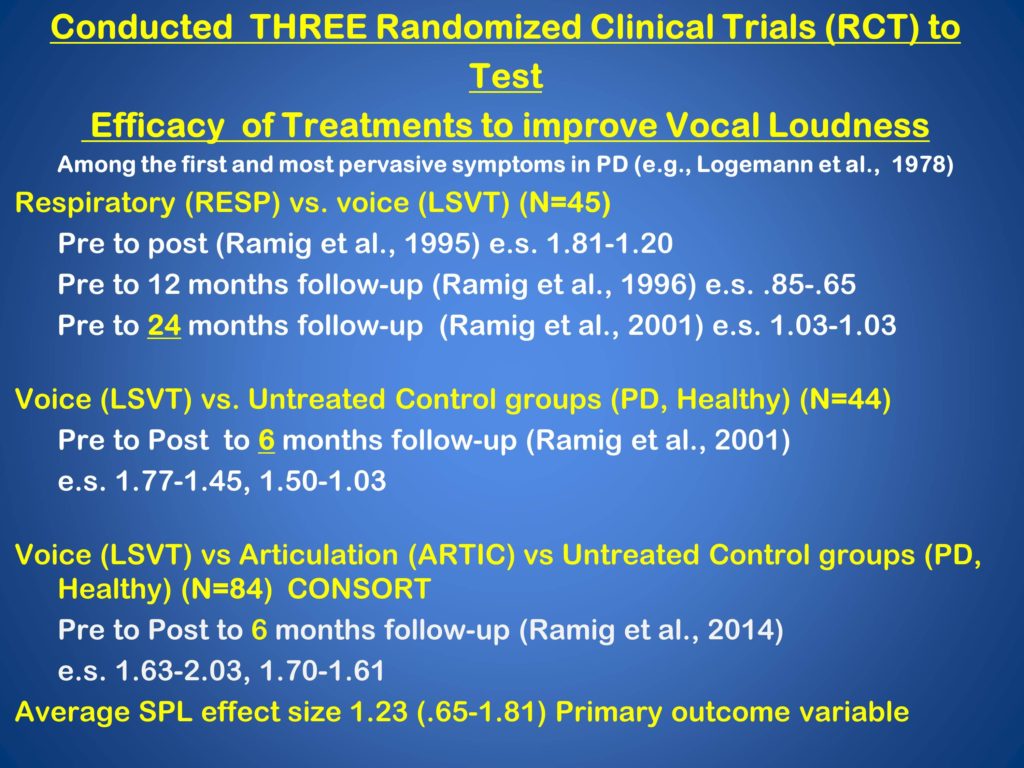
The summary of our randomized controlled trials are here. Over time we have conducted three. We just actually finished the last one a bit ago.
The idea was to test the efficacy of treatments to improve vocal loudness. A primary problem and an early problem in individuals with Parkinson disease. One trial compared high respiratory effort treatment, designed to trigger subglottal pressure, air support. The other focused on high-effort voice treatment. We studied 45 patients, and we studied them out to 24 months treatment.
A second randomized controlled trial looked at high-effort voice treatment, LSVT, compared to untreated control groups, both Parkinson’s and healthy, 44 subjects out to six months.
And the trial we just finished evaluated LSVT compared to high-effort articulation treatment, untreated control groups both Parkinson’s and healthy.
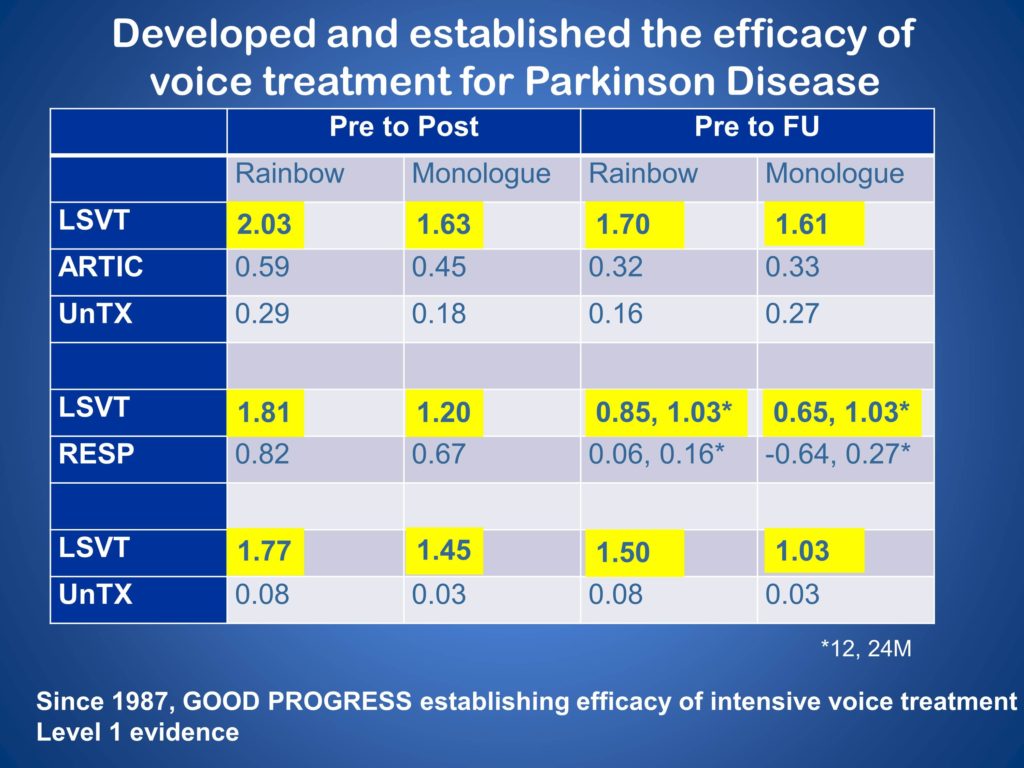
Our average effect sizes are nice, 1.23 over all these studies. This chart summarizes them for the three randomized controlled trials. And we feel like, since 1987, we’ve made good progress in establishing the efficacy of intensive voice treatment.
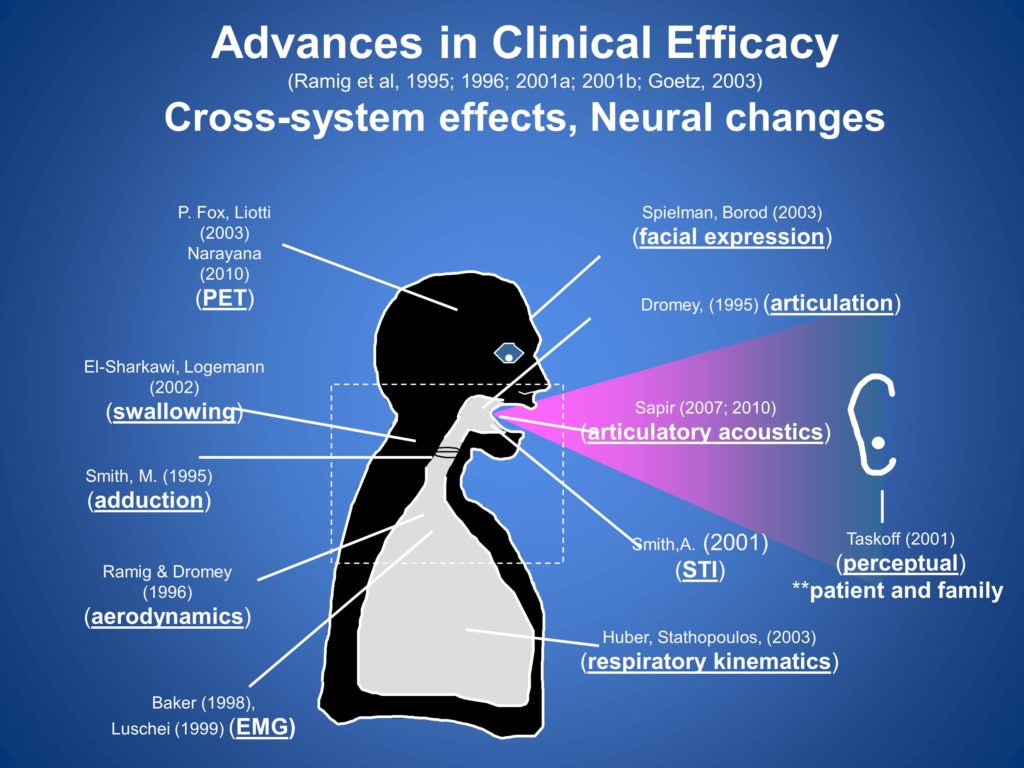
In addition to the primary outcome variable of SPL, as a part of our funded studies, we looked at underlying mechanisms. We wanted to understand what is happening in the subsystems of speech production when we do these various treatments, cross-system effects, as well as neural changes.
In particular, and all these are published, so should you have a boring night some night and want to read these studies feel free to do so.
The exciting approach we’re able to take with these variables, we personally cannot be experts on all of them, but we collect them in a central laboratory all blinded and they are sent off to experts in their own research laboratory to do blinded analyses based on the protocol that we collected. So it’s a very nice approach to be able to look at more variables in collaboration with our colleagues.
One of particular relevance to what we’ve been talking about here is the actual impact of treatments on patients and family members. So we have nice outcome data in that regard in terms of the external validity of the treatment.
Product Development

So, what is our product of this treatment? And I’m moving in the direction of thinking of this as a product. It is a standard research-based behavioral treatment protocol. And here it is. The intensity is standardized across all of the treatments. It is different from previous voice treatments, and that is an important point to be made, we have a single target of vocal loudness. The mode of delivery is intensive and high effort. And we work on variables such as sensory challenges and cuing problems that patients with Parkinson disease have that make generalization of treatment effects really difficult.
This is different from typical speech treatment in the past which may be multiple targets, take a deep breath, over articulate, slow down, be loud, now walk out the door and attempt to use all those changes in your speech production. Perhaps one or two times a week instead of intensive high-effort delivery. And primarily focused on the motor system. So this was a very novel approach to the delivery of speech treatment in Parkinson disease, and it was specifically based on the motor, sensory and neuropsychological challenges that we knew existed in the population.

Pre-Treatment
Therapist: Have you noticed any changes in your speech or your voice that you would associate with Parkinson’s?
Shirley: Yes, I don’t speak loud enough sometimes.
Therapist: Yes, anything else?
Shirley: Hoarse?
Therapist: Anything else?
Shirley: I stutter, which I never did before.
Therapist: Do this for me if you would. Take a deep breath, and say “ah” for as long as you can.
Shirley: aaaaaaaahhhhhh [9 seconds]
Therapist: Good. Would you say Parkinson disease has caused you to talk less?
Shirley: Yes.
Therapist: Because?
Shirley: Because I stutter and I can’t be heard. If there’s noise in the house, like when the kids come over. Nobody pays attention to me because they can’t hear me. Until I get mad and then yell.
Post-Treatment
Therapist: Take a deep breath and say “ah” for as long as you can.
Shirley: AAAAAAAAHHHHHHHH [21 seconds]
Therapist: Have you noticed changes in your speech or your voice as a result of the speech therapy?
Shirley: Oh, yes.
Therapist: What have you noticed?
Shirley: I talk louder. I think louder. I’m going to sing with the Sons of Pioneers one of these days with my voice.
Therapist: Good for you, that’s excellent. What practicing do you do at home?
Shirley: My “ahs” my Is and my lows. And I read the mail out loud.
Therapist: Excellent. Do you feel like practicing helps?
Shirley: Oh yes.
Therapist: So what do you do when you want to be as easy to understand as possible?
Shirley: Think Loud.
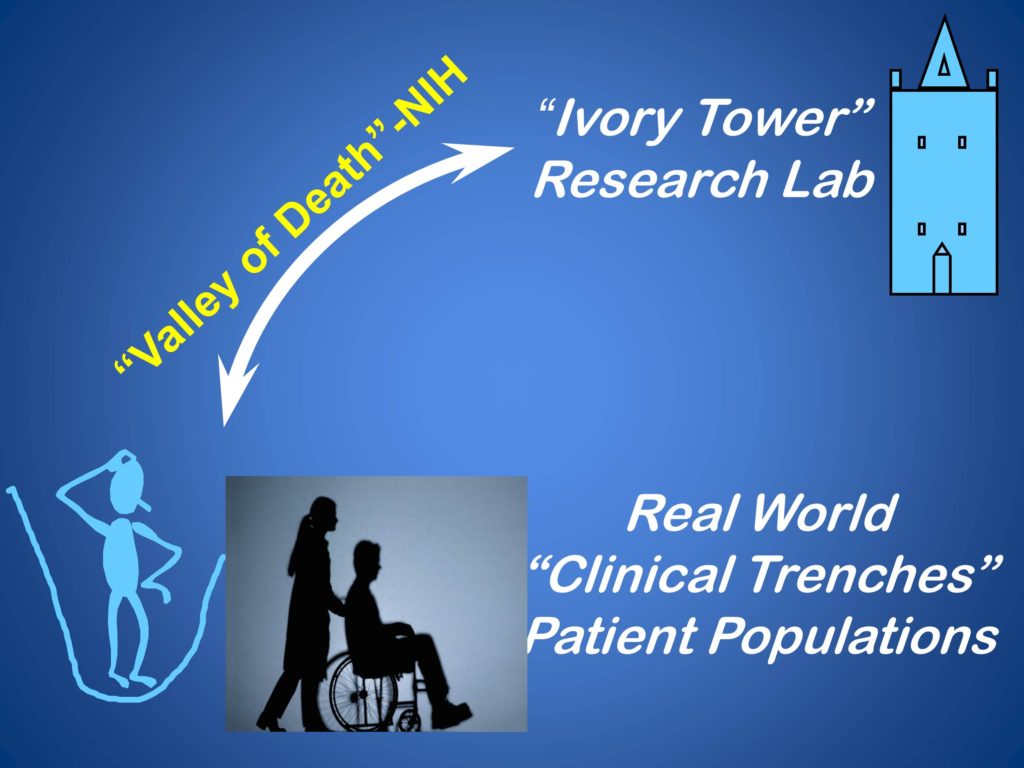
Dissemination and Implementation Pathway
I’m going to walk you through our phases of our best efforts in our attempt to do this, and then tell you where we are.
The Situation: Zero Awareness
We now have the evidence that speech treatment can work and last in Parkinson’s.
We have zero awareness in patients, clinicians, neurologists, and healthcare professionals in the Parkinson community. Nobody knows about it. And even worse than that, Parkinson neurologists are trained that speech therapy doesn’t work for Parkinson’s, changes in the speech treatment room in Parkinson’s disappear on the way to the parking lot. That is published in the literature.
So there we are, what are we going to do?
The Traditional Approach, Part I: Publishing a Guidebook
Well, we are going to go the traditional route. We are going to write a book, and we’re going to say, “Everybody read this book.” — yeah, that’s going to happen — “and implement this treatment.”
Well, what happened? Clinicians were reading it. They were only implementing parts of the protocol. This is where the terror came in, that I talked to you about this morning. We were terrified we were going to lose treatment fidelity with this treatment that could have such a difference in the lives of patients with Parkinson’s. Clearly writing a book and hoping clinicians would read it and doing exactly what we had done in our research just wasn’t going to happen.
So, what should we do?
The Traditional Approach, Part II: Talking to Tech Transfer
Well, again, kind of a traditional approach. We went to our tech transfer office in Boulder, and we said, “Can you help us protect this treatment we have?”
Tech transfer could not get their head around a behavioral treatment. They’re like, “Behavioral treatment? We don’t want it.” We’re like, “Oh no.” We kept thinking, imagine if we invented a drug treatment that had this dramatic effect on a behavior. A pharma would have come along, licensed it, scaled it up, disseminated it, and it would have been out there helping patients.
Well, we weren’t that, so we said, what do we do?
It’s really good we didn’t hear — like we heard yesterday — that developers make poor disseminators. Because we might have stopped at that moment and said, enough with that, we’re going to go back to another research topic.
A New Approach, Part I: Trademarks and Change Management
Be we didn’t. We got bold. And we trademarked the name internationally. We had to have an entity that held the name, so we developed the LSVT Foundation. And we required training and certification for the use of the name LSVT.
Whoa did this require change management. Furthermore, many elements of the treatment flew in the face of conventional speech therapy. And, no participatory action research occurred because we developed this all based on the underlying physiology and in a research lab.
So, let me just tell you a few of the issues we encountered. The clinical world was not ready for a standardized, evidence-based therapy for Parkinson disease. This was wildly novel.
Nobody could believe that a standard protocol, one size fits all, could be implemented in that population.
Intensive dosage? The dosage was 16 hours a week in a one-time-a-week world. Heavens no. And finally, this was the one that was really traumatic for a while, I come from the world of voice, and we were teaching patients to increase vocal loudness which everybody is trained is a hyperfunctional behavior.
So there we sat, with all these challenges, but guess what: We kept doing trainings.
And guess what happened. Clinicians began adopting the protocol because they saw the impact on their patients. And I think that’s a theme we have heard through other interventions as well. When you see an impact of an intervention that’s what motivates the clinical population to pay attention to it.
In addition, we were fortunate, because evidence-based practice started coming to the forefront and we had a lot of evidence for the treatment, and also we trained the clinicians on the underlying mechanism of treatment-related change in the trainings so they start relaxing about this idea that you’re trashing their voice. That was good.
The other exciting thing that happened that really was a game changer, was the world of exercise science came forward with all these amazing data from animal studies documenting the biological basis of exercise. Not just exercise, but intensity of exercise. We were thinking, “High dose, are we ever going to get this implemented?” With these data it’s undeniable high dosage is absolutely essential.
So what happened is the demand for training and treatment increased nationally and internationally. Suddenly people are wanting to learn how to do this treatment. In addition we did some small single-subject and group designs on MS, stroke and cerebral palsy which drove additional interest.
A New Approach, Part II: Adaptation and LSVT BIG
Then came the development of the allied physical therapy called LSVT BIG. And there’s lots to say about BIG, but I’m just going to show you a patient example so you can get an idea. This is also delivered exactly the same as LSVT, intensive, high-effort. But amplitude is scaled up across the body, not just the voice. And you’ll see a quick clip to get an idea.
Referenced clip begins at 17 minutes, 16 seconds of the presentation video, see: https://youtu.be/_zmBC_czzHU?t=17m16s
This guy that you’re going to see is 71. He’s had Parkinson’s for four years. So here he is pre-BIG and here he is post. Again, it’s one month, 16 sessions. And he’s trained in an intensive exercise program with many elements, but his fundamental, generalizable treatment target is “move big.”
As you may know, there are many, many physical therapies today for people with Parkinson’s disease which is so exciting. BIG is just one of them, but it is exactly tailored to the underlying basis of Parkinson’s.
A New Approach, Part III: Accessibility and Growth through Technology
 And so, here we are: We see the power of these interventions. We see how they can make a positive impact on Parkinson’s. We want to facilitate growth and accessibility for patients and clinicians globally. But we do the math, as you may have.
And so, here we are: We see the power of these interventions. We see how they can make a positive impact on Parkinson’s. We want to facilitate growth and accessibility for patients and clinicians globally. But we do the math, as you may have.
One clinician can treat seven patients per month. That would be seven hours a day of LSVT. There are 6 million individuals with Parkinson’s. That’s like 90,000 years before you’re able to give this treatment to all the patients that need it.
In addition, how in the world are we going to train all these speech clinicians, right? It’s fun to go around the world, but come on, really.
So, technology has to be our friend. Both for training and treatment.
We were fortunate to receive funding from Michael J. Fox foundation and NIH to develop a software program that in addition to collecting within-treatment data, it’s a perceptive software program that walks patients through the entire treatment program. It was recently published in AJSLP by my wonderful colleague Angela Halpern, demonstrating patients could use this for half of their treatment sessions. So we can double now the number of patients we can see. In addition, we all know the great advances in telemedicine, which also makes intensive dosage feasible. Again, some positive adaptations.
We also really, in all honesty, wanted to understand. Can we sustain and increase the growth of this entity that will outlast us? I mean, we’re not getting any younger. We want to keep this positive impact going.
So again, what to do?
A New Approach, Part IV: Entrepreneurial Business Model
 The next phase. We transitioned to a business model. We founded the for-profit LSVT Global. This allowed us a mechanism for software commercialization for LSVT companion, as well as development of the online training and certification.
The next phase. We transitioned to a business model. We founded the for-profit LSVT Global. This allowed us a mechanism for software commercialization for LSVT companion, as well as development of the online training and certification.
So now we’re back to the tech transfer office, and our buddies understand us a little bit more now because we’ve got software. And this was a good thing.
In addition, the entrepreneurial center got all excited about it, so then it was like, “Oh boy, we’re getting some help. Maybe someone will teach us and help us and whatever.” We submitted some product and process patents, which is the sort of patent for a program.
Maybe some of you have lived in this world, but under the leadership of the tech transfer office we had a series of CEOs who tried to help us, and we tried to help them. Lots of legal teams, business plans, investment pitches, the whole tamale. But … no success in the shark tank.
We totally failed, and oh the stories I could tell.
So what do we do now?
A New Approach, Part V: Organic Growth Business Model
 Let’s do an organic growth business. We know the business. We know the product. We know the market. Let’s just do it.
Let’s do an organic growth business. We know the business. We know the product. We know the market. Let’s just do it.
Still, our main goal is maintain fidelity of training and treatment, increase accessibility. We now know we need to do more things to sustain clinicians’ ability to deliver the treatment. And we also know based on the literature, plus our own experience, patients need to have lifelong practice. How are we going to do that to help them?
So we went to SBIR funding, technology partners, and we enhance our clinician database on our website so patients can go find a clinician where they live, yadda, yadda, yadda.
What do we see for the future? Harness clinician power globally. Grow technology to support life-long training and treatment. We have an integrated training LSVT HYBRID that does voice and body at the same time, and LOUD for LIFE and BIG for LIFE are approaches to keep patients practices forever.
Partner with healthcare companies — we have done that. And some training institutions, so we can actually get students trained in LSVT as well. We have reimbursement for these trainings, but use research data to support more rehabilitative reimbursement.
And continued dissemination. We must continue to disseminate all the time.
Evolution: Using a Business Model to Impact Fidelity and Accessibility
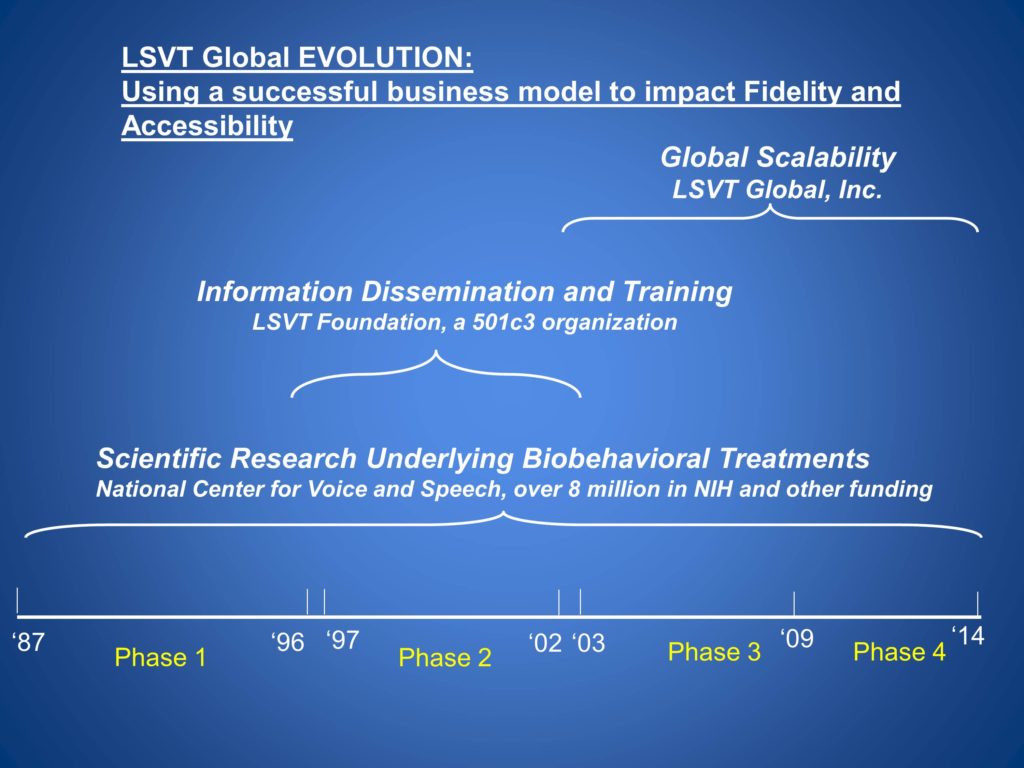
So this was our evolution across time, from the very beginning to date. We still have scientific research underlying our behavioral treatments. And once we learn something new, this gets translated to clinicians quickly. We have a foundation for information dissemination and training, and have moved to LSVT Global to help our efforts in global scalability.
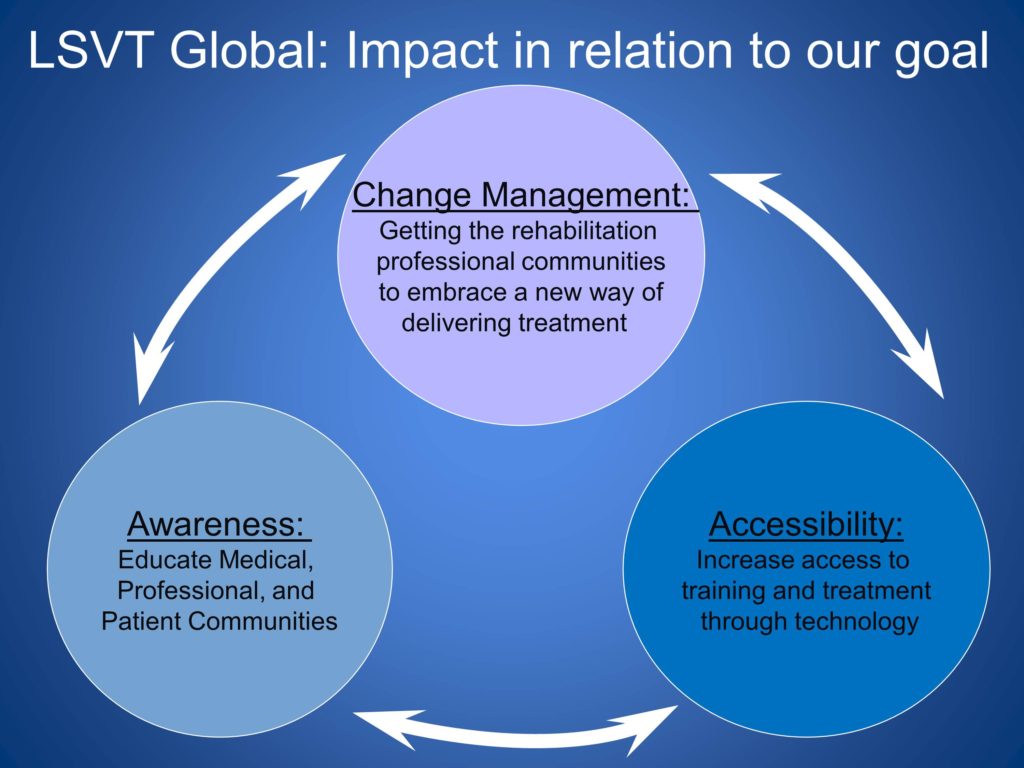
So overall, we’ve moved in all of these fronts, which are very related. We’ve done change management, getting the rehabilitation communities to embrace a new way of delivering treatment. Increase accessibility to treatment through technology. And continuous work on increasing awareness. As we all know, we have to be helping our medical colleagues understand the value of rehabilitation in managing patients.

Today, some people would say that LSVT is the global standard for speech treatment in Parkinson’s. The National Institutes for Clinical Excellence have embraced it as a treatment that any person for Parkinson’s in the UK that wants it has to be able to get it.
We have dug in on maintaining treatment fidelity. We fundamentally believe that has been the secret to success to getting this treatment out.
And we globalized, standardized treatment. LSVT is LSVT is LSVT all around the world. And the value of standardized training. And we feel that has allowed us to maintain the quality of treatment outcomes.
Summary

So, what have we done?
Training. When we began, we had zero. We now have 15,000 trained clinicians in 54 countries, online training, etc. The idea is to continue to sustain and enhance the fidelity of treatment.
We estimate today, and admittedly it’s an estimation, 150,000 treated patients globally. And a variety of other advancements as well.
So we have made progress, and that’s a good thing.
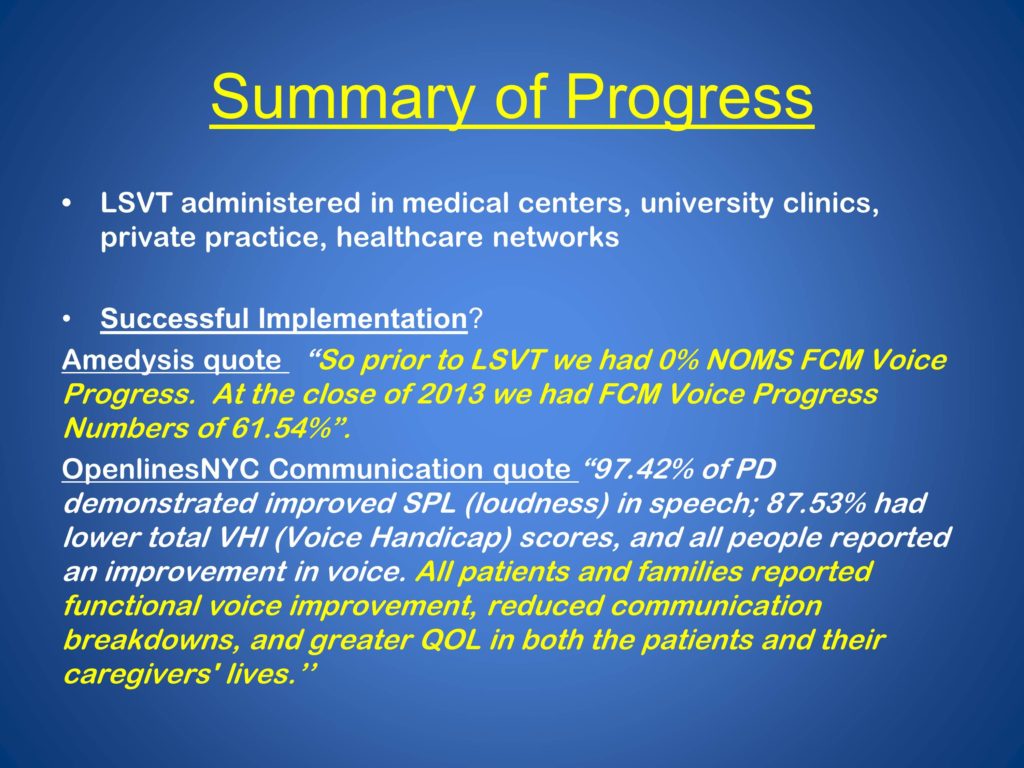
We need to make more.
LSVT today is administered in medical centers, university clinics, private practice and healthcare networks. We have data from a variety of sources. I just want to highlight one from Amedisis, a company we partner with, who trains all their speech clinicians in LSVT. The CEO said, “So prior to LSVT we had 0% NOMS FCM Voice Progress. At the close of 2013 we had FCM Voice Progress Numbers of 61.54%” So we are impacting the real clinical world in measure that people in the world are documenting.
Business Model Ingredients
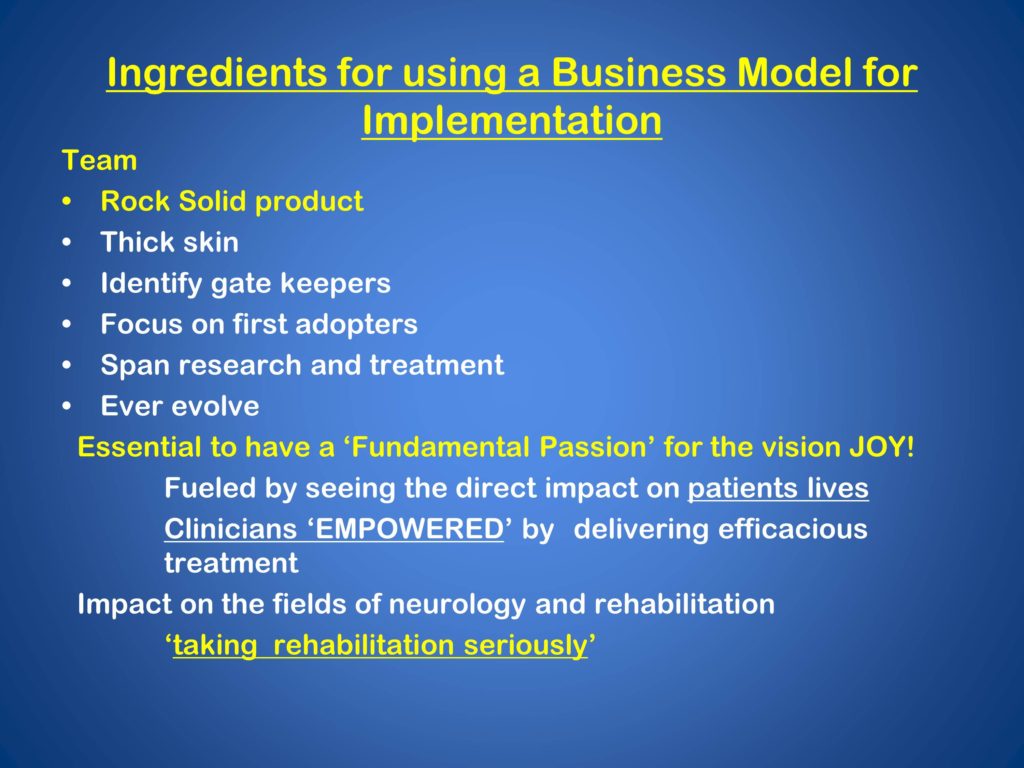
What do we think was essential to taking this business model approach to implementation?
Number one, the team. The team has to be the best team in the whole world, and has to just believe, believe, believe. Because the amount of midnight oil and toil and whatever is astonishing. That’s huge.
A rock solid product. Absolutely rock solid product. Issues related to that are key.
Think skin.
Identify gate keepers. In our world, the MDs, once they got on board with the idea the exercise was valuable for Parkinson’s — huge advancement.
Focus on first adopters.
Continuously span research and treatment. We do that all the time. Something new comes up in the lab, we translate it to our clinicians. We teach them. They give us feedback. It’s just a continuous escalation.
And ever evolve.
And we always like to say, it’s essential to have a fundamental passion for the vision. Where does the joy come from? We were talking today about, was this too hard or whatever? Being fueled by seeing the direct impact on patients’ lives — but one honestly unexpected outcome is how thrilling it is to see clinicians empowered when they can deliver efficacious treatments. That is just so satisfying.
What we also like is the impact on the fields of neurology and rehabilitation. We are positioned now to be able to say to neurologists: Take rehabilitation seriously. You can medicate your patients and get an effect up to here. We can top that off with behavioral intervention and that’s a good thing.
 This world is not for the faint of heart.
This world is not for the faint of heart.
Change management, translating research treatment to real world scope of practice requires seeing the world through many eyes. And it’s amazing.
Open your mind and be persistent.
Recognize your competence, and trust yourself. That’s a really key element. Nobody knows your world better than you do. And that’s important.
Don’t stop believing, as Journey said. Our field is rich with potential to dramatically improve quality of life.
And when we hear our patients say things like, “My voice is alive again.”; “I can talk to my grandchildren.”; “I feel like my old self.”; “LVST BIG has changed my life…the impact is beyond a miracle.”; and “I am confident I can communicate.” that satisfies us a practicing clinicians. And that’s a good thing.
I want to show this video. This patient had LSVT seven or eight years ago, and she had both BIG and LOUD. And sometimes when Parkinson patients retire, they become very creative. And one of the things she chose to do was write rap songs. And so she’s going to do a rap for you.
This person is named Sharon. She was treated in Tucson, Arizona, by my colleague Cynthia Fox. And she came to the office and offered this to us.
Referenced clip begins at 27 minutes, 56 seconds of the presentation video, see: https://youtu.be/_zmBC_czzHU?t=27m56s
This is what LSVT sounds like eight years later.
Yo homies! Listen up.
I’ve got my bling, and I’ve got my cap. I’m the L-DOPA diva and I’m here to rap. When I say “say what, say what” You say, “Kick Parkinson’s butt.”
Say what, say what?
<Kick Parkinson’s butt!>
Say what, say what?
<Kick Parkinson’s butt!>
When your voice is scratchy and your volume is low, and people say speak louder, wherever you go. The unlikely treatment is to bellow. Endless aaahs. Over and over without any pause. Dr. Fox is relentless. She says in terms gruff, “If it sounds too loud, it’s just loud enough.” When I say “Think loud” you say “AAAAAH.”
Think loud.
<AAAAHHHHHHHH>
Well our hands are shaking, and our pace is slow. We have hallucinations and our feet won’t go. But if we can laugh at ourselves, without much fuss, we might have Parkinson’s, but it’ll never have us! When I say “Parkinson’s” you say “Don’t give in.”
Parkinson’s
<Don’t give in!>
Parkinson’s
<Don’t give in!>
Parkinson’s
<Don’t give in!>
I’m down with that.
Selected References
Ramig, L., Sapir, S., Countryman, S., Pawlas, A., O’Brien, C., Hoehn, M. & Thompson, L. (2001). Intensive voice treatment (LSVT®) for patients with Parkinson’s disease: A 2 year follow up. Journal of Neurology, Neurosurgery & Psychiatry, 71(4), 493–498 [Article]
Ramig, L. O., Sapir, S., Fox, C. & Countryman, S. (2001). Changes in vocal loudness following intensive voice treatment (LSVT®) in individuals with Parkinson’s disease: A comparison with untreated patients and normal age‐matched controls. Movement Disorders, 16(1), 79–83 [Article] [PubMed]
Sapir, S., Ramig, L. O. & Fox, C. M. (2011). Intensive voice treatment in Parkinson’s disease: Lee Silverman voice treatment. Expert Reviews in Neurotherapeutics, 11(6),
Liotti, M., Ramig, L., Vogel, D., New, P., Cook, C., Ingham, R., Ingham, J. & Fox, P. (2003). Hypophonia in Parkinson’s disease neural correlates of voice treatment revealed by PET. Neurology, 60(3), 432–440 [Article] [PubMed]
Narayana, S., Fox, P. T., Zhang, W., Franklin, C., Robin, D. A., Vogel, D. & Ramig, L. O. (2010). Neural correlates of efficacy of voice therapy in Parkinson’s disease identified by performance–correlation analysis. Human Brain Mapping, 31(2), 222–236 [PubMed]
Fox, C. M. & Boliek, C. A. (2012). Intensive voice treatment (LSVT LOUD) for children with spastic cerebral palsy and dysarthria. Journal of Speech, Language, and Hearing Research, 55(3), 930–945 [Article]
Ebersbach, G., Ebersbach, A., Edler, D., Kaufhold, O., Kusch, M., Kupsch, A. & Wissel, J. (2010). Comparing exercise in Parkinson’s disease—the Berlin LSVT BIG study. Movement Disorders, 25(12), 1902–1908 [Article] [PubMed]
Halpern, A. E., Ramig, L. O., Matos, C. E., Petska-Cable, J. A., Spielman, J. L., Pogoda, J. M., Gilley, P. M., Sapir, S., Bennett, J. K. & Mcfarland, D. H. (2012). Innovative technology for the assisted delivery of intensive voice treatment (LSVT® LOUD) for Parkinson disease. American Journal of Speech-Language Pathology, 21(4), 354–367 [Article] [PubMed]





